what helps to give different membrane compartments their own unique surface identity?
The routes that lead inward from the cell surface to lysosomes showtime with the process of endocytosis, by which cells take up macromolecules, particulate substances, and, in specialized cases, fifty-fifty other cells. In this process, the textile to be ingested is progressively enclosed past a pocket-size portion of the plasma membrane, which first invaginates and so pinches off to class an endocytic vesicle containing the ingested substance or particle. Ii main types of endocytosis are distinguished on the basis of the size of the endocytic vesicles formed. One blazon is chosen phagocytosis ("cellular eating"), which involves the ingestion of large particles, such as microorganisms or dead cells via big vesicles called phagosomes (by and large >250 nm in diameter). The other blazon is pinocytosis ("cellular drinking"), which involves the ingestion of fluid and solutes via small pinocytic vesicles (about 100 nm in diameter). Nigh eucaryotic cells are continually ingesting fluid and solutes by pinocytosis; large particles are most efficiently ingested by specialized phagocytic cells.

Specialized Phagocytic Cells Can Ingest Big Particles
Phagocytosis is a special form of endocytosis in which large particles such equally microorganisms and expressionless cells are ingested via big endocytic vesicles called phagosomes. In protozoa, phagocytosis is a course of feeding: large particles taken up into phagosomes end upwards in lysosomes, and the products of the subsequent digestive processes pass into the cytosol to exist utilized as food. Even so, few cells in multicellular organisms are able to ingest such large particles efficiently. In the gut of animals, for instance, the particles of nutrient are cleaved downward extracellularly and their hydrolysis products are imported into cells.
Phagocytosis is important in most animals for purposes other than nutrition, and information technology is mainly carried out past specialized cells—so-called professional phagocytes. In mammals, three classes of white claret cells act every bit professional phagocytes—macrophages, neutrophils, and dendritic cells. These cells all develop from hemopoietic stem cells (discussed in Affiliate 22), and they defend us against infection past ingesting invading microorganisms. Macrophages also have an important role in scavenging senescent cells and cells that have died by apoptosis (discussed in Chapter 17). In quantitative terms, the latter function is past far the almost important: our macrophages phagocytose more than 1011 senescent ruddy blood cells in each of united states every solar day, for instance.
Whereas the endocytic vesicles involved in pinocytosis are small and compatible, phagosomes have diameters that are determined past the size of the ingested particle, and they tin exist almost as large as the phagocytic cell itself (Figure xiii-39). The phagosomes fuse with lysosomes inside the cell, and the ingested material is then degraded. Any boxy substances will remain in lysosomes, forming rest bodies. Some of the internalized plasma membrane components never achieve the lysosome, because they are retrieved from the phagosome in transport vesicles and returned to the plasma membrane.
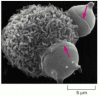
Figure thirteen-39
Phagocytosis by a macrophage. A scanning electron micrograph of a mouse macrophage phagocytosing two chemically contradistinct red claret cells. The red arrows indicate to edges of thin processes (pseudopods) of the macrophage that are extending as collars to engulf (more...)
To be phagocytosed, particles must kickoff bind to the surface of the phagocyte. Nonetheless, not all particles that demark are ingested. Phagocytes take a variety of specialized surface receptors that are functionally linked to the phagocytic machinery of the cell. Unlike pinocytosis, which is a constitutive process that occurs continuously, phagocytosis is a triggered process, requiring that receptors be activated that transmit signals to the cell interior and initiate the response. The best-characterized triggers are antibodies, which protect u.s.a. past bounden to the surface of infectious microorganisms to form a coat in which the tail region of each antibody molecule, chosen the Fc region, is exposed on the exterior (discussed in Chapter 24). This antibody coat is recognized by specific Fc receptors on the surface of macrophages and neutrophils, whose bounden induces the phagocytic cell to extend pseudopods that engulf the particle and fuse at their tips to form a phagosome (Figure 13-xl).

Figure 13-40
Phagocytosis by a neutrophil. An electron micrograph of a neutrophil phagocytosing a bacterium, which is in the process of dividing. (Courtesy of Dorothy F. Bainton, Phagocytic Mechanisms in Wellness and Disease. New York: Intercontinental Book Corporation, (more than...)
Several other classes of receptors that promote phagocytosis have been characterized. Some recognize complement components, which collaborate with antibodies in targeting microbes for destruction (discussed in Chapter 25). Others direct recognize oligosaccharides on the surface of certain microorganisms. Nonetheless others recognize cells that have died past apoptosis. Apoptotic cells lose the asymmetric distribution of phospholipids in their plasma membrane. As a effect, negatively charged phosphatidylserine, which is normally confined to the cytosolic leaflet of the lipid bilayer, is now exposed on the outside of the prison cell, where it triggers the phagocytosis of the expressionless cell.
Remarkably, macrophages will also phagocytose a variety of inanimate particles—such as glass, latex chaplet, or asbestos fibers—yet they practise not phagocytose live animal cells. Information technology seems that living brute cells brandish "don't-eat-me" signals in the form of cell-surface proteins that bind to inhibiting receptors on the surface of macrophages. The inhibitory receptors recruit tyrosine phosphatases that antagonize the intracellular signaling events required to initiate phagocytosis, thereby locally inhibiting the phagocytic procedure. Thus phagocytosis, like many other jail cell processes, depends on a balance betwixt positive signals that actuate the procedure and negative signals that inhibit it.
Pinocytic Vesicles Form from Coated Pits in the Plasma Membrane
Virtually all eucaryotic cells continually ingest bits of their plasma membrane in the grade of pocket-sized pinocytic (endocytic) vesicles, which are afterward returned to the cell surface. The rate at which plasma membrane is internalized in this process of pinocytosis varies between cell types, but information technology is usually surprisingly large. A macrophage, for example, ingests 25% of its own volume of fluid each hour. This means that it must ingest 3% of its plasma membrane each minute, or 100% in about half an hr. Fibroblasts endocytose at a somewhat lower charge per unit (i% per infinitesimal), whereas some amoebae ingest their plasma membrane even more rapidly. Since a cell'southward surface surface area and volume remain unchanged during this process, it is clear that the same corporeality of membrane that is being removed by endocytosis is being added to the cell surface by exocytosis, the converse procedure, as we discuss later. In this sense, endocytosis and exocytosis are linked processes that tin can exist considered to plant an endocytic-exocytic cycle.
The endocytic part of the cycle oftentimes begins at clathrin-coated pits. These specialized regions typically occupy about two% of the total plasma membrane area. The lifetime of a clathrin-coated pit is curt: within a minute or and so of being formed, it invaginates into the cell and pinches off to class a clathrin-coated vesicle (Figure xiii-41). It has been estimated that nearly 2500 clathrin-coated vesicles leave the plasma membrane of a cultured fibroblast every minute. The coated vesicles are even more transient than the coated pits: inside seconds of being formed, they shed their coat and are able to fuse with early endosomes. Since extracellular fluid is trapped in clathrin-coated pits as they invaginate to course coated vesicles, whatsoever substance dissolved in the extracellular fluid is internalized—a process called fluid-stage endocytosis.

Effigy xiii-41
The formation of clathrin-coated vesicles from the plasma membrane. These electron micrographs illustrate the likely sequence of events in the formation of a clathrin-coated vesicle from a clathrin-coated pit. The clathrin-coated pits and vesicles shown (more...)
Not All Pinocytic Vesicles Are Clathrin-coated
In addition to clathrin-coated pits and vesicles, there are other, less well-understood mechanisms past which cells can course pinocytic vesicles. One of these pathways initiates at caveolae (from the Latin for "little cavities"), originally recognized by their ability to transport molecules across endothelial cells, which form the inner lining of blood vessels. Caveolae are present in the plasma membrane of most cell types, and in some of these they are seen as deeply invaginated flasks in the electron microscope (Effigy thirteen-42). They are idea to form from lipid rafts, which are patches of the plasma membrane that are especially rich in cholesterol, glycosphingolipids, and GPI-anchored membrane proteins (see Effigy 12-57). The major structural poly peptide in caveolae is caveolin, a multipass integral membrane protein that is a fellow member of a heterogeneous protein family.

Figure thirteen-42
Caveolae in the plasma membrane of a fibroblast. (A) This electron micrograph shows a plasma membrane with a very high density of caveolae. Note that no cytosolic coat is visible. (B) This rapid-freeze deep-compose image demonstrates the feature "cauliflower" (more...)
In contrast to clathrin-coated and COPI- or COPII-coated vesicles, caveolae are thought to invaginate and collect cargo proteins by virtue of the lipid composition of the calveolar membrane, rather than past the assembly of a cytosolic protein coat. Caveolae pinch off from the plasma membrane and can deliver their contents either to endosome-like compartments or (in a process called transcytosis, which is discussed later) to the plasma membrane on the reverse side of a polarized cell. Some fauna viruses also enter cells in vesicles derived from caveolae. The viruses are first delivered to an endosome-similar compartment, from where they are moved to the ER. In the ER, they extrude their genome into the cytosol to starting time their infectious wheel. It remains a mystery how textile endocytosed in caveolae-derived vesicles can cease upwardly in and then many dissimilar locations in the cell.
Cells Import Selected Extracellular Macromolecules by Receptor-mediated Endocytosis
In most beast cells, clathrin-coated pits and vesicles provide an efficient pathway for taking up specific macromolecules from the extracellular fluid. In this process, chosen receptor-mediated endocytosis, the macromolecules bind to complementary transmembrane receptor proteins, accrue in coated pits, and so enter the cell as receptor-macromolecule complexes in clathrin-coated vesicles (see Figure 13-41). Receptor-mediated endocytosis provides a selective concentrating machinery that increases the efficiency of internalization of particular ligands more than than a hundredfold, so that fifty-fifty minor components of the extracellular fluid can exist specifically taken up in large amounts without taking in a correspondingly big volume of extracellular fluid. A particularly well-understood and physiologically important example is the process whereby mammalian cells accept up cholesterol.
Many animals cells take up cholesterol through receptor-mediated endocytosis and, in this mode, acquire most of the cholesterol they require to brand new membrane. If the uptake is blocked, cholesterol accumulates in the blood and tin contribute to the formation in blood vessel walls of atherosclerotic plaques, deposits of lipid and gristly tissue that tin crusade strokes and heart attacks by blocking blood flow. In fact, it was through a study of humans with a strong genetic predisposition for atherosclerosis that the mechanism of receptor-mediated endocytosis was showtime clearly revealed.
Most cholesterol is transported in the blood every bit cholesteryl esters in the form of lipid-protein particles known equally low-density lipoproteins (LDL) (Figure thirteen-43). When a cell needs cholesterol for membrane synthesis, it makes transmembrane receptor proteins for LDL and inserts them into its plasma membrane. One time in the plasma membrane, the LDL receptors diffuse until they acquaintance with clathrin-coated pits that are in the process of forming (Figure 13-44A). Since coated pits constantly pinch off to form coated vesicles, any LDL particles spring to LDL receptors in the coated pits are quickly internalized in coated vesicles. Later on shedding their clathrin coats, the vesicles deliver their contents to early endosomes, which are located near the cell periphery. Once the LDL and LDL receptors run across the low pH in the endosomes, LDL is released from its receptor and is delivered via late endosomes to lysosomes. At that place the cholesteryl esters in the LDL particles are hydrolyzed to free cholesterol, which is now available to the prison cell for new membrane synthesis. If too much gratis cholesterol accumulates in a jail cell, the cell shuts off both its own cholesterol synthesis and the synthesis of LDL receptor proteins, so that it ceases either to brand or to take up cholesterol.
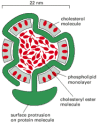
Effigy thirteen-43
A low-density lipoprotein (LDL) particle. Each spherical particle has a mass of 3 × 106 daltons. It contains a core of about 1500 cholesterol molecules esterified to long-chain fatty acids that is surrounded by a lipid monolayer equanimous of well-nigh (more than...)
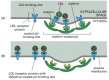
Figure 13-44
Normal and mutant LDL receptors. (A) LDL receptor proteins bounden to a coated pit in the plasma membrane of a normal cell. The human LDL receptor is a single-laissez passer transmembrane glycoprotein composed of about 840 amino acids, only 50 of which are on the (more than...)
This regulated pathway for the uptake of cholesterol is disrupted in individuals who inherit lacking genes encoding LDL receptor proteins. The resulting high levels of blood cholesterol predispose these individuals to develop atherosclerosis prematurely, and many dice at an early age of centre attacks resulting from coronary artery affliction. In some cases, the receptor is defective altogether. In others, the receptors are lacking—in either the extracellular binding site for LDL or the intracellular binding site that attaches the receptor to the glaze of a clathrin-coated pit (see Effigy 13-44B). In the latter case, normal numbers of LDL-binding receptor proteins are nowadays, but they fail to go localized in the clathrin-coated regions of the plasma membrane. Although LDL binds to the surface of these mutant cells, it is not internalized, directly demonstrating the importance of clathrin-coated pits in the receptor-mediated endocytosis of cholesterol.
More than than 25 different receptors are known to participate in receptor-mediated endocytosis of different types of molecules, and they all apparently use the same clathrin-coated-pit pathway. Many of these receptors, like the LDL receptor, enter coated pits irrespective of whether they have jump their specific ligands. Others enter preferentially when spring to a specific ligand, suggesting that a ligand-induced conformational change is required for them to activate the point sequence that guides them into the pits. Since near plasma membrane proteins fail to go concentrated in clathrin-coated pits, the pits must function as molecular filters, preferentially collecting certain plasma membrane proteins (receptors) over others.
Signal peptides guide transmembrane proteins into clathrin-coated pits by binding to the adaptins. Despite a mutual role, their amino acrid sequences vary. A common endocytosis signal consists of simply four amino acids Y-10-X-Ψ, where Y is tyrosine, X any polar amino acid, and Ψ a hydrophobic amino acid. This short peptide, which is shared by many receptors, binds straight to one of the adaptins in clathrin-coated pits. By contrast, the cytosolic tail of the LDL receptor contains a unique indicate (Asn-Pro-Val-Tyr) that plain binds to the same adaptin protein.
Electron-microscope studies of cultured cells exposed simultaneously to different labeled ligands demonstrate that many kinds of receptors tin can cluster in the aforementioned coated pit. The plasma membrane of one clathrin-coated pit can probably accommodate upwards to g receptors of assorted varieties. Although all of the receptor-ligand complexes that utilize this endocytic pathway are apparently delivered to the same endosomal compartment, the subsequent fates of the endocytosed molecules vary, as we discuss next.
Endocytosed Materials That Are Not Retrieved From Endosomes End Upwards in Lysosomes
The endosomal compartments of a cell can be complex. They can be made visible in the electron microscope by adding a readily detectable tracer molecule, such as the enzyme peroxidase, to the extracellular medium and leaving the cells for various lengths of fourth dimension to take information technology upwardly past endocytosis. The distribution of the molecule later on its uptake reveals the endosomal compartments equally a gear up of heterogeneous, membrane-enclosed tubes extending from the periphery of the jail cell to the perinuclear region, where it is often close to the Golgi apparatus. Two sequential sets of endosomes tin be readily distinguished in such labeling experiments. The tracer molecule appears within a minute or then in early endosomes, just beneath the plasma membrane. Afterward five–15 minutes, it moves to late endosomes, shut to the Golgi apparatus and almost the nucleus. Early and late endosomes differ in their protein compositions; they are associated with unlike Rab proteins, for example.
As mentioned earlier, the interior of the endosomal compartment is kept acidic (pH ~vi) by a vacuolar H+ ATPase in the endosomal membrane that pumps H+ into the lumen from the cytosol. In general, later endosomes are more acidic than early endosomes. This acidic environment has a crucial role in the office of these organelles.
Nosotros accept already seen how endocytosed materials that reach the tardily endosomes become mixed with newly synthesized acid hydrolases and end upward being degraded in lysosomes. Many molecules, however, are specifically diverted from this journey to destruction. They are recycled instead from the early on endosomes back to the plasma membrane via ship vesicles. Simply molecules that are not retrieved from endosomes in this way are delivered to lysosomes for degradation.
Specific Proteins Are Removed from Early Endosomes and Returned to the Plasma Membrane
The early endosomes form a compartment that acts as the main sorting station in the endocytic pathway, simply every bit the cis and trans Golgi networks serve this function in the biosynthetic-secretory pathway. In the acidic environment of the early endosome, many internalized receptor proteins change their conformation and release their ligand, simply as the M6P receptors unload their cargo of acid hydrolases in the fifty-fifty more acidic late endosomes. Those endocytosed ligands that dissociate from their receptors in the early endosome are ordinarily doomed to destruction in lysosomes, along with the other soluble contents of the endosome. Some other endocytosed ligands, however, remain bound to their receptors, and thereby share the fate of the receptors.
The fates of the receptor proteins—and of any ligands remaining bound to them—vary according to the specific type of receptor. (1) Most receptors are recycled and render to the same plasma membrane domain from which they came; (2) some proceed to a unlike domain of the plasma membrane, thereby mediating a process called transcytosis; and (iii) some progress to lysosomes, where they are degraded (Figure xiii-45).
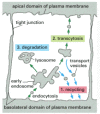
Figure 13-45
Possible fates for transmembrane receptor proteins that have been endocytosed. Three pathways from the endosomal compartment in an epithelial cell are shown. Retrieved receptors are returned (one) to the same plasma membrane domain from which they came (more...)
The LDL receptor follows the first pathway. It dissociates from its ligand LDL in the early endosome and is recycled to the plasma membrane for reuse, leaving the discharged LDL to be carried to lysosomes (Figure 13-46). The recycling vesicles bud from long, narrow tubules that extend from the early endosomes. It is probable that the geometry of these tubules helps the sorting process. Considering tubules accept a big membrane area enclosing a small volume, membrane proteins tend to accumulate in that location. Ship vesicles that render material to the plasma membrane begin budding from the tubules, but tubular portions of the early on endosome also compression off and fuse with one another to form recycling endosomes, a way-station for the traffic between the early endosomes and the plasma membrane. During this procedure, the tubules and then the recycling endosome continuously shed vesicles that return to the plasma membrane.
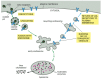
Figure 13-46
The receptor-mediated endocytosis of LDL. Note that the LDL dissociates from its receptors in the acidic environs of the endosome. After a number of steps (run into Figure 13-48), the LDL ends up in lysosomes, where it is degraded to release free cholesterol. (more...)
The transferrin receptor follows a similar recycling pathway, only it also recycles its ligand. Transferrin is a soluble poly peptide that carries fe in the blood. Cell-surface transferrin receptors evangelize transferrin with its jump iron to early endosomes past receptor-mediated endocytosis. The depression pH in the endosome induces transferrin to release its leap fe, but the iron-free transferrin itself (chosen apotransferrin) remains spring to its receptor. The receptor-apotransferrin complex enters the tubular extensions of the early on endosome and from there is recycled back to the plasma membrane (Figure 13-47). When the apotransferrin returns to the neutral pH of the extracellular fluid, it dissociates from the receptor and is thereby freed to choice upward more iron and begin the cycle again. Thus, transferrin shuttles back and forth between the extracellular fluid and the endosomal compartment, avoiding lysosomes and delivering the iron that cells need to grow to the cell interior.
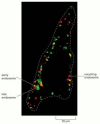
Figure 13-47
Sorting of membrane proteins in the endocytic pathway. Transferrin receptors mediate food uptake and constitutively cycle betwixt endosomes and the plasma membrane. By contrast, opioid receptors are signaling receptors that—after ligand binding—are (more...)
The 2nd pathway that endocytosed receptors tin follow from endosomes is taken both by opioid receptors (see Figure thirteen-47) and by the receptor that binds epidermal growth gene (EGF). EGF is a modest, extracellular signal protein that stimulates epidermal and various other cells to divide. Unlike LDL receptors, EGF receptors accumulate in clathrin-coated pits only afterwards binding EGF, and most of them do not recycle but are degraded in lysosomes, along with the ingested EGF. EGF bounden therefore commencement activates intracellular signaling pathways and and then leads to a decrease in the concentration of EGF receptors on the cell surface, a process called receptor down-regulation that reduces the cell's subsequent sensitivity to EGF (discussed in Chapter 15).
Multivesicular Bodies Form on the Pathway to Late Endosomes
It is all the same uncertain how endocytosed molecules move from the early to the late endosomal compartment so equally to finish up in lysosomes. A current view is that portions of the early endosomes migrate slowly along microtubules toward the cell interior, shedding tubules of material to be recycled to the plasma membrane. The migrating endosomes enclose large amounts of invaginated membrane and internally pinched-off vesicles and are therefore called multivesicular bodies (Effigy 13-48). It is unknown whether multivesicular bodies eventually fuse with a belatedly endosomal compartment or if they fuse instead with each other to become late endosomes. At the finish of this pathway, the late endosomes are converted to lysosomes as a result of their fusion with hydrolase-bearing transport vesicles from the trans Golgi network and their increased acidification (Figure 13-49).

Figure thirteen-48
Electron micrograph of a multivesicular body in a plant cell. The large amount of internal membrane will be delivered to the vacuole, the establish equivalent of the lysosome, for digestion.
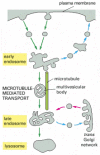
Figure 13-49
The endocytic pathway from the plasma membrane to lysosomes. Maturation from early to late endosomes occurs through the formation of multivesicular bodies, which contain large amounts of invaginated membrane and internal vesicles (hence their proper noun). These (more...)
The multivesicular bodies carry specific endocytosed membrane proteins that are to be degraded but exclude others that are to be recycled. Equally part of the protein-sorting process, specific proteins—for example, the occupied EGF receptor described previously—selectively partition to the invaginating membrane of the multivesicular bodies (Figure 13-50). In this mode, the receptors, as well equally any signaling proteins strongly jump to them, are rendered fully accessible to the digestive enzymes that will degrade them (see Figure 13-50).

Effigy thirteen-50
The sequestration of endocytosed proteins into internal membranes of multivesicular bodies. Eventually, all of the internal membranes produced past the invaginations shown are digested past proteases and lipases in lysosomes. The invagination is essential (more...)
Membrane proteins that are sorted into the internal membrane vesicles of a multivesicular torso are first covalently modified with the small poly peptide ubiquitin. Unlike multi-ubiquitylation which typically targets substrate proteins for degradation in proteasomes (discussed in Chapter six), ubiquitin tagging for sorting into the internal membrane vesicles of a multivesicular body requires the improver of just a single ubiquitin molecule that is added to activated receptors while notwithstanding at the plasma membrane. The ubiquitin tag facilitates the uptake of the receptors into endocytic vesicles and is then recognized again by proteins that mediate the sorting process into the internal membrane vesicles of multivesicular bodies. In addition, membrane invagination in multivesicular bodies is regulated past a lipid kinase that phosphorylates phosphatidylinositol. The phosphorylated caput groups of these lipids are thought to serve every bit docking sites for the proteins that mediate the invagination procedure. Local modification of lipid molecules is thus another style in which specific membrane patches tin be induced to change shape and destiny.
In addition to endocytosed membrane proteins, multivesicular bodies also contain nigh of the soluble content of early endosomes destined for digestion in lysosomes.
Macromolecules Can Exist Transferred Across Epithelial Cell Sheets by Transcytosis
Some receptors on the surface of polarized epithelial cells transfer specific macromolecules from i extracellular space to another by transcytosis (Figure thirteen-51). These receptors are endocytosed and so follow a pathway from endosomes to a dissimilar plasma membrane domain (see Effigy thirteen-46). A newborn rat, for example, obtains antibodies from its mother's milk (which help protect it confronting infection) past transporting them across the epithelium of its gut. The lumen of the gut is acidic, and, at this low pH, the antibodies in the milk bind to specific receptors on the apical (absorptive) surface of the gut epithelial cells. The receptor-antibody complexes are internalized via clathrin-coated pits and vesicles and are delivered to early endosomes. The complexes remain intact and are retrieved in ship vesicles that bud from the early endosome and subsequently fuse with the basolateral domain of the plasma membrane. On exposure to the neutral pH of the extracellular fluid that bathes the basolateral surface of the cells, the antibodies dissociate from their receptors and eventually enter the newborn'southward bloodstream.
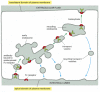
Figure 13-51
Transcytosis. Recycling endosomes form a way-station on the transcytotic pathway. In the example shown here, an antibody receptor on a gut epithelial cell binds antibiotic and is endocytosed, eventually carrying the antibody to the basolateral plasma membrane (more...)
The transcytotic pathway from the early endosome to the plasma membrane is non straight. The receptors first move from the early on endosome to an intermediate endosomal compartment, the recycling endosome described previously (see Figure 13-51). The variety of pathways that unlike receptors follow from endosomes implies that, in add-on to binding sites for their ligands and bounden sites for coated pits, many receptors also possess sorting signals that guide them into the appropriate type of transport vesicle leaving the endosome and thereby to the appropriate target membrane in the jail cell.
A unique property of a recycling endosomes is that the go out of membrane proteins from the compartment can be regulated. Thus, cells can adjust the flux of proteins through the transcytotic pathway according to need. Although the machinery of regulation is uncertain, it allows recycling endosomes an of import office in adjusting the concentration of specific plasma membrane proteins. Fatty cells and musculus cells, for example, contain large intracellular pools of the glucose transporters that are responsible for the uptake of glucose beyond the plasma membrane. These proteins are stored in specialized recycling endosomes until the cell is stimulated by the hormone insulin to increase its rate of glucose uptake. And then transport vesicles bud from the recycling endosome and deliver big numbers of glucose transporters to the plasma membrane, thereby profoundly increasing the rate of glucose uptake into the prison cell (Effigy 13-52).

Figure 13-52
Storage of plasma membrane proteins in recycling endosomes. Recycling endosomes can serve as an intracellular pool for specialized plasma membrane proteins, enabling them to be mobilized when needed. In the example shown hither, insulin bounden to the insulin (more...)
Epithelial Cells Have Ii Distinct Early on Endosomal Compartments Just a Common Late Endosomal Compartment
In polarized epithelial cells, endocytosis occurs from both the basolateral domain and the apical domain of the plasma membrane. Material endocytosed from either domain first enters an early endosomal compartment that is unique to that domain. This arrangement allows endocytosed receptors to exist recycled back to their original membrane domain, unless they contain signals that mark them for transcytosis to the other domain. Molecules endocytosed from either plasma membrane domain that are not retrieved from the early endosomes end up in a common tardily endosomal compartment near the cell eye and are somewhen degraded in lysosomes (Figure 13-53).
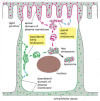
Effigy 13-53
The two distinct early on endosomal compartments in an epithelial cell. The basolateral and the apical domains of the plasma membrane communicate with carve up early endosomal compartments. But endocytosed molecules from both domains that practice non contain (more...)
Whether cells incorporate a few connected or many unconnected endosomal compartments seems to depend on the jail cell blazon and the physiological state of the cell. Like many other membrane-enclosed organelles, endosomes of the same type tin can readily fuse with one another (an example of homotypic fusion, discussed earlier) to create large continuous endosomes.
Summary
Cells ingest fluid, molecules, and particles by endocytosis, in which localized regions of the plasma membrane invaginate and pinch off to course endocytic vesicles. Many of the endocytosed molecules and particles stop upward in lysosomes, where they are degraded. Endocytosis occurs both constitutively and every bit a triggered response to extracellular signals. Endocytosis is so extensive in many cells that a large fraction of the plasma membrane is internalized every hour. To brand this possible, most of the plasma membrane components (proteins and lipid) that are endocytosed are continually returned to the jail cell surface by exocytosis. This large-scale endocytic-exocytic bicycle is mediated largely past clathrin-coated pits and vesicles.
Many cell-surface receptors that demark specific extracellular macromolecules go localized in clathrin-coated pits. As a consequence, they and their ligands are efficiently internalized in clathrin-coated vesicles, a procedure called receptor-mediated endocytosis. The coated endocytic vesicles rapidly shed their clathrin coats and fuse with early on endosomes.
Most of the ligands dissociate from their receptors in the acidic environment of the endosome and eventually cease upward in lysosomes, while well-nigh of the receptors are recycled via ship vesicles back to the cell surface for reuse. But receptor-ligand complexes tin follow other pathways from the endosomal compartment. In some cases, both the receptor and the ligand cease upward beingness degraded in lysosomes, resulting in receptor down-regulation. In other cases, both are transferred to a different plasma membrane domain, and the ligand is thereby released by exocytosis at a surface of the cell unlike from that where it originated, a procedure called transcytosis. The transcytosis pathway includes recycling endosomes, where endocytosed plasma membrane proteins can be stored until they are needed.

Source: https://www.ncbi.nlm.nih.gov/books/NBK26870/
0 Response to "what helps to give different membrane compartments their own unique surface identity?"
Post a Comment